Dementia and Suicidal Behavior a Review of the Literature
Introduction
Suicide is a major public health result and the xiii cause of expiry worldwide. Suicidality can be represented every bit a continuum from suicidal ideation to suicidal act, which includes suicide attempts (SA) and death past suicide. Although SA frequency is higher in young adults (Conejero et al., 2016) and then progressively decreases (Hawton and Harriss, 2008), suicide rates increase with age, reaching the highest level in older adults in near all countries (World Health Organization, 2014). Indeed, suicide rate amongst white men older than 85 years of age was 48.7/100,000 in the Usa in 2004 (more than than four times the national age-adapted rate of 11.1/100,000), and 140/100,000 among men aged or older than 75 in rural China in 1999 (Conwell and Thompson, 2008). Moreover, amidst older people, suicide rate increases with historic period (Shah et al., 2016). The ratio between deliberate self-harm and completed suicide varies from 200 for teenagers to ten for people over 60 (Hawton and Harriss, 2008). In older adults, many conditions have been related to suicide: chronic illnesses, physical disabilities, cancer, social isolation, mental and neurocognitive disorders (Duberstein et al., 2004a,b; Voshaar et al., 2015). Among neurocognitive disorders, the study of the relationships between dementia and suicidal behaviors gave conflicting results. Dementia represents the major cause of autonomy loss in older adults. As a chronic disease, it may induce depressive symptoms and suicidal ideation. Alzheimer's illness (Advertizing), the most frequent cause of dementia, is characterized clinically by cerebral dysfunctions, nigh normally involving episodic retention and behavioral disorders. Advertizing pathogenesis is thought to be driven by pathological aggregation of beta amyloid (Aβ) and tau proteins in the brain (Reitz et al., 2011; Uzun et al., 2011). Aβ deposition seems to exist the first brain lesion, especially in frontal areas. Several techniques are used to assess encephalon amyloid brunt, including brain positron emission tomography (PET) and biomarker quantification in cerebrospinal fluid (CSF) (Uzun et al., 2011). In vivo biomarker quantification could permit the diagnosis of Advertizement at an early stage when only cognitive complaints are present, or even earlier in people at take a chance due to family unit history or harboring the apolipoprotein Eε4 allele (APOE4). AD diagnosis could correspond a critical moment that increases the risk of suicidal ideation and act. In improver, some early on behavioral disorders in Advertizement, such as low and altered controlling related to frontal encephalon lesions, may contribute to increase the suicide gamble. Specifically, information technology is non articulate whether (i) AD increases the risk of suicidal ideation and SA, or the frequency of death past suicide; (ii) the presence of suicidal ideation or SA in older (≥65-year-one-time) people could exist an early sign of AD; and (iii) the amyloid load in frontal areas facilitates SA by modifying the decision-making pathway. In this review, we provide updated findings on the links between suicidal behavior, dementia and encephalon amyloid burden, in club to address these questions. We also hash out the clinical and pathophysiological role of low in the relationship between dementia and suicidal behaviors.
Materials and Methods
We conducted a narrative review of original studies selected from PubMed using the medical bailiwick heading (MeSH) terms ("Suicide" AND "Depression") OR ("Amyloid" OR "Dementia"). Among the 13,732 articles retrieved, we retained 8,921 written in English, after 2000. We excluded comments, books, documents, case reports, preclinical studies and manufactures with no abstruse bachelor. Then, we kept four,755 full-text articles. Finally, we selected 31 articles written in English language that were published from 2000 to 2017 and corresponded to the most representative studies (e.m., in terms of impact factor, sample size, authority of the experts, type of publication, such as meta-analyses/reviews). The listing of references was also reviewed to identify other studies of interest (n = 38). The study catamenia chart is presented in Figure 1.
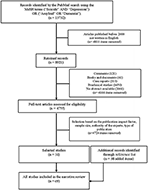
Figure 1. Chart presenting the choice procedure of the studies.
Results and Word
Suicide and Dementia
Clan Between Completed Suicide and Dementia
There are conflicting results in the literature concerning the link between completed suicide and dementia. These discrepancies could be explained by the heterogeneity of the dementia groups, the lack of standardization of the tools used for the diagnosis of dementia and suicidal behaviors, and also the absence of stratification relative to the illness stage. Although authentic histopathological assessment was performed in few works, retrospective studies do not permit the precise categorization of the dementia blazon based merely on the standardized clinical and para-clinical test. Furthermore, most of the studies on suicide and dementia were performed in relatively small clinical samples.
Presence of association
Patients with dementia take a (iii- to 10-fold) college risk to die past suicide, even when taking into account potential misreckoning factors, such as mood disorders (Erlangsen et al., 2008). Amidst the unlike dementia types, patients with Huntington'south illness are particularly at risk (Harris and Barraclough, 1997; Haw et al., 2009), with a charge per unit of completed suicide of virtually 13% (Cummings, 1995). In women, the suicide hazard associated with vascular dementia is significantly lower than that associated with Advertisement (Erlangsen et al., 2008). Advertising pathology (based on examination of hippocampal sections) is more frequent in people older than threescore years of age who committed suicide (n = 28) than in age- and sex-matched controls (n = 56) (Rubio et al., 2001). Specifically, the modified Braak score (reflecting the number of neurofibrillary tangles) was higher in the suicide victims than in controls (Rubio et al., 2001), whereas amyloid load was comparable in both groups.
Moreover, during the early stages of AD, the risk of completed suicide is highest and so decreases (Erlangsen et al., 2008; Cipriani et al., 2013). In the first half dozen months after the diagnosis of dementia, the increased take a chance for completed suicide could be explained by the: (1) awareness of cognitive reject (Serafini et al., 2016) and feeling of burdensomeness toward meaning others; (two) stress induced by the anticipation of autonomy loss and the feeling of harm in daily life functioning (Cipriani et al., 2013); (iii) increased prevalence of comorbid depressive and adjustment disorders (Seyfried et al., 2011; Cipriani et al., 2013; Draper, 2015) (4) effect of potential comorbidities, such as bipolar disorders, substance utilise, and anxiety disorders (Seyfried et al., 2011); (five) even so good cognitive functions at the early stage of affliction that allow patients to plan suicide and complete the suicidal deed; and (6) deficits of executive functions, controlling and inhibition process (Richard-Devantoy et al., 2012, 2016). Other hazard factors for completed suicide have been identified at the early AD stage, such equally late onset of cognitive decline, male gender and high educational level (Cipriani et al., 2013). However, the part of each of these factors has not been validated in patients in whom Advertizement diagnosis was confirmed by neuropathological information or in vivo biomarker quantification. In addition, the clinical characteristics of early stage AD differed amid studies, and no data was available on prodromal AD symptoms.
Absence of clan
Some studies did not detect any additional risk of completed suicide in people with dementia. For instance, a comparison of 85 cases of suicide and 153 living controls older than 65 years establish that the odds ratio for the association with dementia is less than ane (Wærn et al., 2002). No completed suicide was observed among the 104 participants who died (76 were women) during the four-year follow-up of 277 patients with dementia (Harris and Barraclough, 1997). The assay of hippocampal sections of 143 community-dwelling suicide victims anile over 65 years and 59 controls did not highlight any difference in plaque score and neurofibrillary tangle staging (Peisah et al., 2007). The American Psychiatric Association guidelines on the care of patients with dementia take reported no added gamble of suicide in this population "Elderly persons in general and elderly men in particular are at increased chance for suicide, although the diagnosis of dementia is not known to confer added run a risk", p. eighteen (Rabins and McIntyre, 2010). Severe cerebral damage in late stage dementia could protect confronting completed suicide by reducing the chapters to accomplish a suicidal plan (Cipriani et al., 2013).
Clan Between Dementia and Suicide Attempt or Suicidal Ideation
In individuals with dementia, SA rate is lower than one% (Schneider et al., 2001) and fifty-fifty completely absent (retrospective analysis of 148 patients with AD, 24 with vascular dementia and 49 with dementia types) (Draper et al., 1998). It seems that SA occurs in patients with dementia and psychiatric comorbidities (Draper, 2015). In patients older than 65 years of age hospitalized in a psychiatric unit of measurement following SA, SA was positively associated with comorbid psychiatric disorders and history of SA. Moreover, the take chances for suicidal behaviors decreases when cognitive impairment increases, based on to the Mini Mental State Examination (MMSE) score (Osvath et al., 2005).
Suicidal ideation in patients with dementia is rare. Based on the suicide detail (particular iii) of the Hamilton Rating Scale for Depression, x% (n = nine) of 91 patients with probable Advertizing reported hopelessness, merely not suicidal ideation (Harwood and Sultzer, 2002). Lifetime suicidal ideas are not more frequent in patients with dementia than in age-matched not-demented participants, fifty-fifty later on controlling for lifetime major low. In add-on, suicidal ideation and feelings of worthlessness are correlated with the severity of cognitive decline, measured past the MMSE (Heun et al., 2003). In some other study, almost 4% of patients with Advertisement reported suicidal ideation ("wish to dice" by iii.2% and suicidal ideation past 0.9%, according to the Hamilton Rating Scale for Depression) (Draper et al., 1998).
The apparent contradiction between studies showing that dementia could predispose to consummate suicide, especially at an early stage of the disease, and other works reporting that dementia could be associated with a lower gamble for SA or suicidal ideation could partly explained past the bear on of the diagnosis announcement and the long-term management of the illness. This is an important indicate because AD diagnosis could be realized at an early stage and patient instruction and awareness on this consequence is constantly increasing. Therefore, it is crucial to improve our noesis virtually the potential triggering effects of the announcement of a diagnosis of dementia (Mattsson et al., 2010; Mitchell et al., 2013). Although this could have a positive bear upon, such equally deciding to spend more than time with loved ones, forming partnerships with other people with dementia, receiving therapeutic support, improving the quality of patient care (De Lepeleire et al., 2004), the consequence on suicidal ideation/deed needs to be further assessed. To our knowledge, very few studies have directly analyzed the clan between dementia diagnosis disclosure and suicidal behavior. Turnbull et al. (2003) evaluated the attitudes toward Advertizing diagnosis in 200 outpatients older than 65 years of age and institute that 92% wanted to know almost the diagnosis and one.vii% wanted to be told well-nigh the diagnosis of Advert in gild to commit suicide (Turnbull et al., 2003). Withal, the impact of AD diagnosis disclosure on suicide hazard should exist more specifically assessed.
Studies prove a suicidal ideation prevalence between ii.2 and xvi.seven% in Chinese crumbling populations (Simon et al., 2013), and more than 5% in other representative samples of older adults (Barnow and Linden, 2000; Scocco and De Leo, 2002). In our clinical experience, the charge per unit of suicidal ideation and SA in individuals with cerebral complaints after the kickoff visit to a retention eye is lower than 2%. In a feasibility study, we assessed suicidal ideation/act using the C-SSRS questionnaire (Posner et al., 2011) in patients with cerebral complaints at the Memory Resources Research Center of Montpellier from Jan 31, 2016 to July 1, 2017. Among the 1691 participants, only 32 [1.nine%, median age 61 (45–88) years] had at least 1 positive answer to the following 2 questions: "Accept y'all wished y'all were dead or wished you could go to sleep and never wake up?" and "Take you actually idea most committing suicide?" Nosotros classified participants as having moderate suicidal ideation (n = 16, 0.95%) when they gave only one positive respond, and equally having severe suicidal ideation (north = xvi, 0.95%) when they gave 2 positive answers. Amid these 32 participants, 21.8% had past history of SA, 31.2% had a neurodegenerative disorder (AD, frontotemporal dementia, Lewy body disease) and 31.two% had a subjective retention complaint. Amid the participants with negative answers to both questions, only 0.4% reported past history of SA (unpublished data).
Are Suicidal Behavior and Amyloid Associated via Depression?
No report has analyzed the relationship betwixt the underlying pathological process, such as the amyloid load, and suicidal behaviors. Low could induce suicidal ideation and/or SA, only too brain amyloid deposition or changes in the CSF level of amyloid peptides. For instance, in a group of ≥60-yr-old people without dementia, amyloid brunt, measured by PET with 18-F-florbetapir, in the parietal and precuneus cortices was college among people with lifetime diagnosis of major depressive disorder than in controls without low (Wu et al., 2014). These amyloid deposits are related to handling-resistant low (Li et al., 2017), a well-known chance factor of suicidal beliefs (Greden, 2001; Olin et al., 2012). Depression is oftentimes plant in patients with early stage dementia and could exist a event of neurobiological changes in specific brain regions (Andersen et al., 2005). In patients with mild cognitive impairment (MCI), amyloid load has been linked to late-onset depression (Tateno et al., 2015), and to lifetime history of depression (Chung et al., 2015). Depression level has been associated with higher FDDNP binding to amyloid plaques and neurofibrillary tangles (quantified by PET) in the lateral temporal regions in participants with MCI, and with FDDNP bounden in the medial temporal cortex in controls without MCI (Lavretsky et al., 2009). Moreover, FDDNP binding in the posterior cingulate and lateral temporal regions is higher in depressed than healthy controls aged betwixt 60 and 82 years (Kumar et al., 2011). Apathy severity, evaluated with the Apathy Evaluation Scale, has been associated with amyloid load (by FDDNP-PET) in the anterior cingulate cortex in 16 patients with tardily-life depression (Eyre et al., 2017). Conversely, using the Pittsburgh Chemical compound-B (PiB) tracer, findings are controversial (Butters et al., 2008; Madsen et al., 2012; Yasuno et al., 2016). Most studies detected a meaning clan between Aβ burden and depressive symptoms or major depressive episodes; still, some negative findings were as well reported, peradventure due to the small sample size and lack of statistical power. Concerning CSF biomarkers, changes in Aβ42 level or Aβ40/42 ratio accept been related to late-life depression (Nascimento et al., 2015). During a longitudinal follow-up, participants with depression displayed a slightly, but significant lower CSF Aβ42 level than non-depressed individuals (Pomara et al., 2016). CSF Aβ42 may be a state-dependent marking. Indeed, Aβ42 levels are lower in more than severe depression, and the improvement of depressive symptoms is associated with CSF Aβ42 increase (Pomara et al., 2016).
The higher amyloid burden during depression in older people is associated with increased risk to develop Advertizing in the future; notwithstanding, it is difficult to distinguish between depression equally a prodromal manifestation of dementia, or as contained condition. Additional studies with longitudinal design, amyloid PET imaging or CSF amyloid measurements, and depression cess are needed to decide the potential causal relationship. Indeed, low could exist a risk factor (Barnes et al., 2012), or a prodromal manifestation of dementia, specially amyloid-associated low (Lord's day et al., 2008).
Amyloid deposition in the central nervous system has also been linked to serotoninergic dysregulation, dysfunctional stress response, and inflammation of brain regions involved in suicidal vulnerability. Serotoninergic pathways take been involved in Aβ-associated depressive episodes and are impaired past Aβ aggregating in the fundamental nervous organization (Gonzalo-Ruiz et al., 2003). Moreover, the serotoninergic arrangement is contradistinct in AD (Trillo et al., 2013; Ramirez et al., 2014; Verdurand and Zimmer, 2017), and amyloid deposition in the brain impairs the serotoninergic action in animal models (Colaianna et al., 2010; Ledo et al., 2013). Aβ peptides display neurotoxic activeness (Piccinni et al., 2013), and they could also change the brain inflammatory response, which in plough modifies the expression of indoleamine2,3-dioxygenase and impairs serotoninergic manual (Ledo et al., 2013; Mahgoub and Alexopoulos, 2016). Hence, in early on phase Advert, suicidal behavior could be related to alterations of serotoninergic transmission (Madsen et al., 2011), which have been linked to impulsive and aggressive beliefs (Lai et al., 2003; Vermeiren et al., 2014). In add-on, Aβ could impair the stress response (Catania et al., 2009; Morgese et al., 2017) and dysregulate the hypothalamic-pituitary-adrenal axis that has been involved in suicidal vulnerability (Jokinen and Nordström, 2008; Jokinen et al., 2010; Morgese et al., 2014).
Finally, determination-making impairment (i.e., the choice of options with high firsthand advantage, but disadvantageous in the long-term) has been observed in early stage Ad (Gleichgerrcht et al., 2010), peculiarly in patients with amygdala neurodegeneration and altered connectivity to the ventromedial prefrontal cortex. Moreover, impaired controlling has been linked to CSF amyloid level in patients with Lewy torso illness, and to medial orbitofrontal cortex cloudburst (Spotorno et al., 2017). Notwithstanding, to our knowledge, the relationship between decision-making damage and amyloid load in AD has not been evaluated. Impaired controlling has been also involved in the pathophysiology of suicidal behavior in older adults. Using the Cambridge Gamble Task (Clark et al., 2011), a study showed that amongst ≥65-year-old patients, the quality of decision-making was reduced in depressed suicide attempters compared with depressed non-attempters and healthy controls. Altered controlling was associated with perceived poor social problem-solving. In this written report, dumb controlling in older suicide attempters might not be related to the level of impulsivity, but rather to neglected knowledge of probability. Similarly, depression performances at the Iowa Gambling Task have been observed in violent suicide attempters over 65 years of historic period (Wyart et al., 2016). These results heighten the possibility that amyloid deposition could trigger suicidal behavior by altering conclusion-making in patients with early phase Advertizement.
Strengths and Limitations
The nowadays narrative review of the literature provides an updated motion picture of the virtually recent findings concerning the link between suicidal ideation, suicidal behaviors and dementia. However, some limitations should be underlined. First, about of the studies accept been conducted on small population samples with clinical heterogeneity according to the age and the disease stage. Second, a very few studies explored the link between suicide and the different types of dementia. Hence, we rather focused on the link between suicide and AD. Finally, because our aim was to provide an updated overview of the literature, we did not integrated results published before 2000 in our review.
Conclusion
The presented results suggest that at late affliction stages, dementia could protect against suicidal ideation and SA. Conversely, the adventure of complete suicide is increased during the early phase of cognitive pass up. Serious cognitive impairment and decline of executive functions might protect against negative thoughts related to awareness of cognitive disability and confronting suicide planning. Several factors could contribute to increasing the suicide charge per unit post-obit the diagnosis of dementia: (1) the awareness of cognitive pass up and the feeling of burdensomeness toward meaning others, (ii) the anticipation of autonomy loss, (3) the increased prevalence of comorbid mood and adjustment disorders, (4) the still good cognitive functions at the early on stage of disease that allow patients to plan and complete a suicidal act; (v) the deficit of executive functions, determination-making and inhibition procedure.
Nevertheless, such retrospective analyses do not allow highlighting any clear causal human relationship between dementia and suicidal beliefs. Indeed, suicidal acts in older adults may trigger or precipitate cognitive reject by increasing the stress response and activation of the hypothalamic-pituitary-adrenal axis. Nevertheless, some findings suggest that SA or completed suicide in patients with early stage AD could exist a consequence and a complication of the neurocognitive harm.
Amyloid brunt is a potential risk factor for suicide through its clan with depressive symptoms that are frequently observed in the early stage of dementia, and through its effects on various neurobiological pathways (i.e., serotoninergic dysregulation, dysfunctional stress response and brain inflammation) (Figure 2). Some evidences advise that Aβ load directly alters some of the nigh of import neurobiological pathways underlying suicidal behavior. The suicidal behavior observed in older people early after the diagnosis of dementia should encourage research to assess implicit behavioral data in this population in club to improve suicidal behavior prevention.
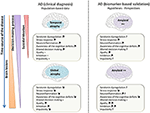
Figure 2. Scheme of the unlike mechanisms that could be involved to explain the relationship betwixt suicidal ideation, decision-making process in Alzheimer's disease (Advertisement) based on clinical diagnosis (part left of the effigy) and in Ad based on biomarkers particularly the amyloid load (role right of the figure). The "↑" is for an increase of the mechanism or blueprint; the "↓" is for a subtract and " = " is related to a stability or an absenteeism of modification of the mechanism or pattern; the "?" is for an unknown data or mechanism.
Author Contributions
IC, SN, JK, EO, AG analyzed the data of the literature and drafted the manuscript. PC, AG conceived and designed the review.
Conflict of Interest Statement
The authors declare that the inquiry was conducted in the absence of any commercial or financial relationships that could be construed every bit a potential conflict of involvement.
References
Andersen, Grand., Lolk, A., Kragh-Sørensen, P., Petersen, Due north. E., and Dark-green, A. (2005). Depression and the hazard of Alzheimer illness. Epidemiology 16, 233. doi: 10.1097/01.ede.0000152116.32580.24
PubMed Abstruse | CrossRef Full Text | Google Scholar
Barnes, D. Due east., Yaffe, Chiliad., Byers, A. 50., McCormick, M., Schaefer, C., and Whitmer, R. A. (2012). Midlife vs. belatedly-life depressive symptoms and risk of dementia: differential effects for Alzheimer affliction and vascular dementia. Arch. Gen. Psychiatry 69, 493–498. doi: 10.1001/archgenpsychiatry.2011.1481
PubMed Abstract | CrossRef Full Text | Google Scholar
Butters, Thou. A., Klunk, Westward. Due east., Mathis, C. A., Price, J. C., Ziolko, S. K., Hoge, J. A., et al. (2008). Imaging Alzheimer pathology in late-life low with PET and Pittsburgh Chemical compound-B. Alzheimer Dis. Assoc. Disord. 22, 261–268. doi: 10.1097/WAD.0b013e31816c92bf
PubMed Abstract | CrossRef Full Text | Google Scholar
Catania, C., Sotiropoulos, I., Silva, R., Onofri, C., Breen, G. C., Sousa, Due north., et al. (2009). The amyloidogenic potential and behavioral correlates of stress. Mol. Psychiatry fourteen, 95–105. doi: ten.1038/sj.mp.4002101
PubMed Abstruse | CrossRef Full Text | Google Scholar
Chung, J. K., Plitman, Due east., Nakajima, S., Chow, T. Westward., Chakravarty, M. Yard., Caravaggio, F., et al. (2015). Lifetime history of depression predicts increased Amyloid-β accumulation in patients with mild cerebral impairment. J. Alzheimers Dis. 45, 907–919. doi: 10.3233/JAD-142931
PubMed Abstract | CrossRef Full Text | Google Scholar
Cipriani, Thousand., Vedovello, M., Lucetti, C., Di Fiorino, A., and Nuti, A. (2013). Dementia and suicidal behavior. Aggress. Violent Behav. 18, 656–659. doi: x.1016/j.avb.2013.07.016
CrossRef Full Text | Google Scholar
Clark, Fifty., Dombrovski, A. Y., Siegle, G. J., Butters, M. A., Shollenberger, C. L., Sahakian, B. J., et al. (2011). Impairment in risk-sensitive decision-making in older suicide attempters with depression. Psychol. Aging 26, 321–330. doi: 10.1037/a0021646
PubMed Abstruse | CrossRef Full Text | Google Scholar
Colaianna, M., Tucci, P., Zotti, M., Morgese, M. K., Schiavone, S., Govoni, Due south., et al. (2010). Soluble beta amyloid(1-42): a critical histrion in producing behavioural and biochemical changes evoking depressive-related state? Br. J. Pharmacol. 159, 1704–1715. doi: 10.1111/j.1476-5381.2010.00669.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Conejero, I., Lopez-Castroman, J., Giner, L., and Baca-Garcia, E. (2016). Sociodemographic antecedent validators of suicidal behavior: a review of recent literature. Curr. Psychiatry Rep. 18:94. doi: ten.1007/s11920-016-0732-z
PubMed Abstract | CrossRef Full Text | Google Scholar
Cummings, J. L. (1995). Behavioral and psychiatric symptoms associated with Huntington's disease. Adv. Neurol. 65, 179–186.
Google Scholar
De Lepeleire, J., Buntinx, F., and Aertgeerts, B. (2004). Disclosing the diagnosis of dementia: the performance of Flemish general practitioners. Int. Psychogeriatr. xvi, 421–428. doi: 10.1017/S1041610204000407
PubMed Abstract | CrossRef Full Text | Google Scholar
Draper, B., MacCuspie-Moore, C., and Brodaty, H. (1998). Suicidal ideation and the "wish to dice" in dementia patients: the office of depression. Historic period Ageing 27, 503–507. doi: 10.1093/ageing/27.4.503
CrossRef Full Text | Google Scholar
Duberstein, P. R., Conwell, Y., Conner, Chiliad. R., Eberly, S., and Caine, E. D. (2004a). Suicide at fifty years of historic period and older: perceived concrete disease, family unit discord and financial strain. Psychol. Med. 34, 137–146. doi: 10.1017/S0033291703008584
PubMed Abstruse | CrossRef Full Text | Google Scholar
Duberstein, P. R., Conwell, Y., Conner, Yard. R., Eberly, S., Evinger, J. S., and Caine, E. D. (2004b). Poor social integration and suicide: fact or artifact? A instance-command study. Psychol. Med. 34, 1331–1337. doi: 10.1017/S0033291704002600
CrossRef Total Text | Google Scholar
Erlangsen, A., Zarit, S. H., and Conwell, Y. (2008). Hospital-diagnosed dementia and suicide: a longitudinal report using prospective, nationwide register data. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 16, 220–228. doi: x.1097/01.JGP.0000302930.75387.7e
PubMed Abstruse | CrossRef Total Text | Google Scholar
Eyre, H. A., Siddarth, P., van Dyk, M., St. Cyr, North., Baune, B. T., Barrio, J. R., et al. (2017). Neural correlates of apathy in late-life depression: a pilot [18 F]FDDNP positron emission tomography written report: imaging apathy in geriatric low. Psychogeriatrics 17, 186–193. doi: ten.1111/psyg.12213
CrossRef Full Text | Google Scholar
Gleichgerrcht, E., Ibáñez, A., Roca, M., Torralva, T., and Manes, F. (2010). Decision-making noesis in neurodegenerative diseases. Nat. Rev. Neurol. 6, 611–623. doi: x.1038/nrneurol.2010.148
PubMed Abstract | CrossRef Full Text | Google Scholar
Gonzalo-Ruiz, A., González, I., and Sanz-Anquela, J. K. (2003). Effects of beta-amyloid protein on serotoninergic, noradrenergic, and cholinergic markers in neurons of the pontomesencephalic tegmentum in the rat. J. Chem. Neuroanat. 26, 153–169. doi: ten.1016/S0891-0618(03)00046-2
PubMed Abstract | CrossRef Total Text | Google Scholar
Greden, J. F. (2001). The burden of disease for treatment-resistant depression. J. Clin. Psychiatry 62, 26–31.
Google Scholar
Harwood, D. Thousand., and Sultzer, D. 50. (2002). "Life is not worth living": hopelessness in Alzheimer's illness. J. Geriatr. Psychiatry Neurol. xv, 38–43. doi: 10.1177/089198870201500108
CrossRef Full Text | Google Scholar
Hawton, K., and Harriss, L. (2008). How often does deliberate self-damage occur relative to each suicide? A study of variations by gender and age. Suicide Life Threat. Behav. 38, 650–660. doi: x.1521/suli.2008.38.6.650
PubMed Abstract | CrossRef Full Text | Google Scholar
Heun, R., Kockler, M., and Ptok, U. (2003). Lifetime symptoms of low in Alzheimer's affliction. Eur. Psychiatry J. Assoc. Eur. Psychiatr. xviii, 63–69. doi: 10.1016/S0924-9338(03)00003-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Jokinen, J., and Nordström, P. (2008). HPA axis hyperactivity as suicide predictor in elderly mood disorder inpatients. Psychoneuroendocrinology 33, 1387–1393. doi: x.1016/j.psyneuen.2008.07.012
PubMed Abstruse | CrossRef Full Text | Google Scholar
Jokinen, J., Ouda, J., and Nordström, P. (2010). Noradrenergic office and HPA axis dysregulation in suicidal behaviour. Psychoneuroendocrinology 35, 1536–1542. doi: 10.1016/j.psyneuen.2010.05.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Kumar, A., Kepe, V., Barrio, J. R., Siddarth, P., Manoukian, V., Elderkin-Thompson, V., et al. (2011). Protein binding in patients with late-life depression. Arch. Gen. Psychiatry 68, 1143–1150. doi: 10.1001/archgenpsychiatry.2011.122
PubMed Abstruse | CrossRef Full Text | Google Scholar
Lai, M. M., Tsang, S. Westward., Francis, P. T., Esiri, Thousand. M., Keene, J., Promise, T., et al. (2003). Reduced serotonin five-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer illness. Encephalon Res. 974, 82–87. doi: x.1016/S0006-8993(03)02554-X
PubMed Abstruse | CrossRef Full Text | Google Scholar
Lavretsky, H., Siddarth, P., Kepe, Five., Ercoli, L. M., Miller, One thousand. J., Burggren, A. C., et al. (2009). Depression and feet symptoms are associated with cerebral FDDNP-PET binding in centre-aged and older non-demented adults. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 17, 493–502. doi: ten.1097/JGP.0b013e3181953b82
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ledo, J. H., Azevedo, E. P., Clarke, J. R., Ribeiro, F. C., Figueiredo, C. P., Foguel, D., et al. (2013). Amyloid-β oligomers link depressive-like behavior and cerebral deficits in mice. Mol. Psychiatry eighteen, 1053–1054. doi: 10.1038/mp.2012.168
PubMed Abstruse | CrossRef Full Text | Google Scholar
Li, P., Hsiao, I.-T., Liu, C.-Y., Chen, C.-H., Huang, S.-Y., Yen, T.-C., et al. (2017). Beta-amyloid degradation in patients with major depressive disorder with differing levels of treatment resistance: a pilot written report. EJNMMI Res. 7:24. doi: 10.1186/s13550-017-0273-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Madsen, K., Hasselbalch, B. J., Frederiksen, K. S., Haahr, M. East., Gade, A., Law, I., et al. (2012). Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol. Aging 33, 2334–2342. doi: 10.1016/j.neurobiolaging.2011.11.021
CrossRef Full Text | Google Scholar
Madsen, K., Neumann, W.-J., Holst, Yard., Marner, Fifty., Haahr, M. T., Lehel, Due south., et al. (2011). Cerebral serotonin 4 receptors and amyloid-β in early on Alzheimer'southward disease. J. Alzheimers Dis. JAD 26, 457–466. doi: x.3233/JAD-2011-110056
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mahgoub, Northward., and Alexopoulos, Yard. S. (2016). The amyloid hypothesis: is in that location a part for anti-amyloid treatment in late-life depression? Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 24, 239–247. doi: 10.1016/j.jagp.2015.12.003
CrossRef Total Text | Google Scholar
Mattsson, N., Brax, D., and Zetterberg, H. (2010). To know or non to know: ethical problems related to early diagnosis of Alzheimer's affliction. Int. J. Alzheimers Dis. 2010:841941. doi: 10.4061/2010/841941
PubMed Abstract | CrossRef Full Text | Google Scholar
Mitchell, G., McCollum, P., and Monaghan, C. (2013). The personal impact of disclosure of a dementia diagnosis: a thematic review of the literature. Br. J. Neurosci. Nurs. 9, 223–228. doi: 10.12968/bjnn.2013.9.five.223
CrossRef Full Text | Google Scholar
Morgese, M. G., Schiavone, S., and Trabace, L. (2017). Emerging role of amyloid beta in stress response: Implication for depression and diabetes. Eur. J. Pharmacol. 817, 22–29. doi: 10.1016/j.ejphar.2017.08.031
PubMed Abstract | CrossRef Total Text | Google Scholar
Morgese, 1000. Yard., Tucci, P., Colaianna, 1000., Zotti, Thousand., Cuomo, V., Schiavone, S., et al. (2014). Modulatory activity of soluble beta amyloid on HPA axis function in rats. Curr. Pharm. Des. 20, 2539–2546. doi: 10.2174/13816128113199990500
PubMed Abstract | CrossRef Full Text | Google Scholar
Nascimento, K. G. F., practice Silva, Grand. P., Malloy-Diniz, Fifty. F., Butters, M. A., and Diniz, B. Southward. (2015). Plasma and cerebrospinal fluid amyloid-β levels in late-life low: a systematic review and meta-analysis. J. Psychiatr. Res. 69, 35–41. doi: x.1016/j.jpsychires.2015.07.024
PubMed Abstract | CrossRef Full Text | Google Scholar
Olin, B., Jayewardene, A. Grand., Bunker, Thousand., and Moreno, F. (2012). Mortality and suicide risk in treatment-resistant depression: an observational study of the long-term impact of intervention. PLoS ONE 7:e48002. doi: 10.1371/periodical.pone.0048002
PubMed Abstract | CrossRef Total Text | Google Scholar
Osvath, P., Kovacs, A., Voros, V., and Fekete, Southward. (2005). Gamble factors of attempted suicide in the elderly: the office of cerebral harm. Int. J. Psychiatry Clin. Pract. 9, 221–225. doi: x.1080/13651500510029020
PubMed Abstract | CrossRef Full Text | Google Scholar
Peisah, C., Snowdon, J., Gorrie, C., Kril, J., and Rodriguez, M. (2007). Investigation of Alzheimer'southward affliction-related pathology in community domicile older subjects who committed suicide. J. Affect. Disord. 99, 127–132. doi: x.1016/j.jad.2006.08.030
PubMed Abstract | CrossRef Full Text | Google Scholar
Piccinni, A., Origlia, Northward., Veltri, A., Vizzaccaro, C., Marazziti, D., Vanelli, F., et al. (2013). Neurodegeneration, β-amyloid and mood disorders: state of the art and future perspectives. Int. J. Geriatr. Psychiatry 28, 661–671. doi: 10.1002/gps.3879
PubMed Abstract | CrossRef Full Text | Google Scholar
Pomara, N., Bruno, D., Osorio, R. S., Reichert, C., Nierenberg, J., Sarreal, A. S., et al. (2016). Country-dependent alterations in cerebrospinal fluid Aβ42 levels in cognitively intact elderly with belatedly-life major depression: Neuroreport 27, 1068–1071. doi: 10.1097/WNR.0000000000000658
PubMed Abstract | CrossRef Total Text | Google Scholar
Posner, Grand., Dark-brown, G. K., Stanley, B., Brent, D. A., Yershova, K. V., Oquendo, 1000. A., et al. (2011). The Columbia–suicide severity rating scale: initial validity and internal consistency findings from iii multisite studies with adolescents and adults. Am. J. Psychiatry 168, 1266–1277. doi: ten.1176/appi.ajp.2011.10111704
PubMed Abstract | CrossRef Total Text | Google Scholar
Rabins, P. V., and McIntyre, J. South. (2010). Treatment of Patients With Alzheimer's Disease and Other Dementias. American psychiatric association, Guidelines.
PubMed Abstract
Ramirez, Yard. J., Lai, M. K., Tordera, R. K., and Francis, P. T. (2014). Serotonergic therapies for cognitive symptoms in Alzheimer's disease: rationale and electric current condition. Drugs 74, 729–736. doi: 10.1007/s40265-014-0217-5
PubMed Abstruse | CrossRef Full Text | Google Scholar
Richard-Devantoy, S., Jollant, F., Kefi, Z., Turecki, Grand., Olié, J. P., Annweiler, C., et al. (2012). Deficit of cognitive inhibition in depressed elderly: a neurocognitive marker of suicidal hazard. J. Affect. Disord. 140, 193–199. doi: x.1016/j.jad.2012.03.006
PubMed Abstruse | CrossRef Full Text | Google Scholar
Rubio, A., Vestner, A. L., Stewart, J. Chiliad., Forbes, N. T., Conwell, Y., and Cox, C. (2001). Suicide and Alzheimer's pathology in the elderly: a case–control written report. Biol. Psychiatry 49, 137–145. doi: 10.1016/S0006-3223(00)00952-5
PubMed Abstract | CrossRef Total Text | Google Scholar
Schneider, B., Maurer, Thousand., and Fröhlich, L. (2001). Demenz und suizid. Fortschritte Neurol. Psychiatr. 69, 164–169. doi: 10.1055/s-2001-12693
CrossRef Full Text | Google Scholar
Scocco, P., and De Leo, D. (2002). One-year prevalence of death thoughts, suicide ideation and behaviours in an elderly population. Int. J. Geriatr. Psychiatry 17, 842–846. doi: ten.1002/gps.691
PubMed Abstruse | CrossRef Total Text | Google Scholar
Serafini, G., Calcagno, P., Lester, D., Girardi, P., Amore, M., and Pompili, M. (2016). Suicide run a risk in Alzheimer'southward illness: a systematic review. Curr. Alzheimer Res. 13, 1083–1099. doi: 10.2174/1567205013666160720112608
PubMed Abstract | CrossRef Total Text | Google Scholar
Seyfried, 50. S., Kales, H. C., Ignacio, R. Five., Conwell, Y., and Valenstein, M. (2011). Predictors of suicide in patients with dementia. Alzheimers Dement. J. Alzheimers Assoc. seven, 567–573. doi: x.1016/j.jalz.2011.01.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Shah, A., Bhat, R., Zarate-Escudero, Due south., DeLeo, D., and Erlangsen, A. (2016). Suicide rates in 5-yr age-bands after the historic period of sixty years: the international landscape. Aging Ment. Health 20, 131–138. doi: 10.1080/13607863.2015.1055552
PubMed Abstract | CrossRef Full Text | Google Scholar
Simon, Chiliad., Chang, E.-South., Zeng, P., and Dong, 10. (2013). Prevalence of suicidal ideation, attempts, and completed suicide rate in chinese crumbling populations: a systematic review. Arch. Gerontol. Geriatr. 57, 250–256. doi: 10.1016/j.archger.2013.05.006
PubMed Abstract | CrossRef Full Text | Google Scholar
Spotorno, N., McMillan, C. T., Irwin, D. J., Clark, R., Lee, East. B., Trojanowski, J. Q., et al. (2017). Decision-making deficits associated with amyloidosis in lewy body disorders. Front. Hum. Neurosci. x:693. doi: 10.3389/fnhum.2016.00693
PubMed Abstract | CrossRef Total Text | Google Scholar
Sun, 10., Steffens, D. C., Au, R., Folstein, Grand., Summergrad, P., Yee, J., et al. (2008). Amyloid-associated depression: a prodromal depression of Alzheimer affliction? Arch. Gen. Psychiatry 65, 542–550. doi: 10.1001/archpsyc.65.5.542
PubMed Abstruse | CrossRef Total Text | Google Scholar
Tateno, A., Sakayori, T., Higuchi, Thou., Suhara, T., Ishihara, Thou., Kumita, S., et al. (2015). Amyloid imaging with [(18)F]florbetapir in geriatric depression: early-onset versus belatedly-onset. Int. J. Geriatr. Psychiatry thirty, 720–728. doi: 10.1002/gps.4215
CrossRef Full Text | Google Scholar
Trillo, L., Das, D., Hsieh, West., Medina, B., Moghadam, S., Lin, B., et al. (2013). Ascending monoaminergic systems alterations in Alzheimer's disease. Translating bones science into clinical care. Neurosci. Biobehav. Rev. 37, 1363–1379. doi: 10.1016/j.neubiorev.2013.05.008
PubMed Abstruse | CrossRef Full Text | Google Scholar
Turnbull, Q., Wolf, A. M., and Holroyd, S. (2003). Attitudes of elderly subjects toward "truth telling" for the diagnosis of Alzheimer's disease. J. Geriatr. Psychiatry Neurol. 16, 90–93. doi: 10.1177/0891988703016002005
PubMed Abstract | CrossRef Total Text | Google Scholar
Uzun, S., Kozumplik, O., and Folnegović-Smalc, 5. (2011). Alzheimer's dementia: current data review. Coll. Antropol. 35, 1333–1337.
PubMed Abstract | Google Scholar
Verdurand, M., and Zimmer, 50. (2017). Hippocampal 5-HT1A receptor expression changes in prodromal stages of Alzheimer's disease: benign or deleterious? Neuropharmacology 123, 446–454. doi: 10.1016/j.neuropharm.2017.06.021
CrossRef Full Text | Google Scholar
Vermeiren, Y., Van Dam, D., Aerts, T., Engelborghs, S., and De Deyn, P. P. (2014). Monoaminergic neurotransmitter alterations in postmortem brain regions of depressed and aggressive patients with Alzheimer's illness. Neurobiol. Aging 35, 2691–2700. doi: 10.1016/j.neurobiolaging.2014.05.031
PubMed Abstract | CrossRef Full Text | Google Scholar
Voshaar, R. C., van der Veen, D. C., Kapur, N., Hunt, I., Williams, A., and Pachana, Northward. A. (2015). Suicide in patients suffering from late-life anxiety disorders; a comparison with younger patients. Int. Psychogeriatr. 27, 1197–1205. doi: 10.1017/S1041610215000125
PubMed Abstract | CrossRef Total Text
Wærn, G., Runeson, B. Southward., Allebeck, P., Beskow, J., Rubenowitz, E., Skoog, I., et al. (2002). Mental disorder in elderly suicides: a case-command study. Am. J. Psychiatry 159, 450–455. doi: 10.1176/appi.ajp.159.3.450
PubMed Abstruse | CrossRef Full Text | Google Scholar
World Health Organization (2014). Preventing Suicide: A Global Imperative. Geneva: Earth Health Organisation.
Wu, 1000.-Y., Hsiao, I.-T., Chen, C.-Due south., Chen, C.-H., Hsieh, C.-J., Wai, Y.-Y., et al. (2014). Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 41, 714–722. doi: x.1007/s00259-013-2627-0
PubMed Abstruse | CrossRef Full Text | Google Scholar
Wyart, Chiliad., Jaussent, I., Ritchie, K., Abbar, M., Jollant, F., and Courtet, P. (2016). Iowa gambling task functioning in elderly persons with a lifetime history of suicidal acts. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 24, 399–406. doi: 10.1016/j.jagp.2015.12.007
PubMed Abstract | CrossRef Total Text | Google Scholar
Yasuno, F., Kazui, H., Morita, Due north., Kajimoto, Thou., Ihara, M., Taguchi, A., et al. (2016). High amyloid-β deposition related to depressive symptoms in older individuals with normal noesis: a pilot study. Int. J. Geriatr. Psychiatry 31, 920–928. doi: 10.1002/gps.4409
PubMed Abstruse | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fnins.2018.00371/full
0 Response to "Dementia and Suicidal Behavior a Review of the Literature"
Post a Comment